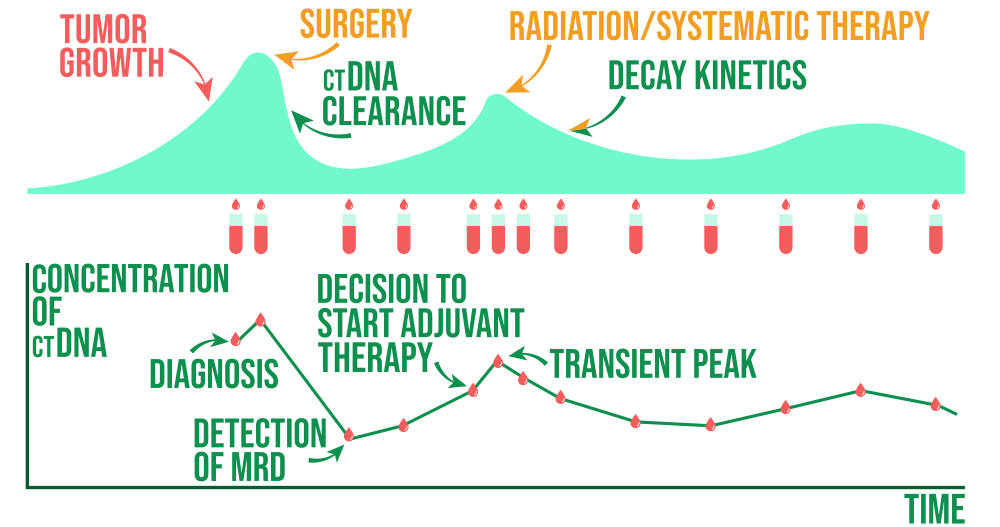
MRD for treatment development
Cell-free tumor load measuring helps fill an unmet clinical need for real-time, noninvasive detection of tumor response to targeted therapies prior to radiographic assessment by offering a novel method for analyzing longitudinal changes in circulating tumor DNA (ctDNA) during systemic treatment.
New opportunities for individualized cancer diagnosis and treatment in various types of tumors are made possible by ctDNA analysis. Minimum Disease (MRD) and disease recurrence are closely related, which allows identify particular genetic and molecular alterations as new MRD detection targets using ctDNA. MRD is a promising prognostic marker to identify people who may benefit from treatment and are at higher risk of recurrence.
Sampling a patient's plasma in a treatment setting can enable the detection of ctDNA and the potential selection of a patient population harboring genetic or epigenetic alterations that might be susceptible to being targeted by a particular drug under investigation.
FDA suggests using MRD
The US Food and Drug Administration (FDA) in May 2022 finalized guidance to help sponsors planning to use minimal residual disease (MRD) as a biomarker in clinical trials for treating specific hematologic malignancies.
The guidance technology that can detect the persistence of malignancy at, what FDA says are, "orders of magnitude below the limit of conventional morphologic detection," which has a threshold limit of one tumor cell in 100 cells.

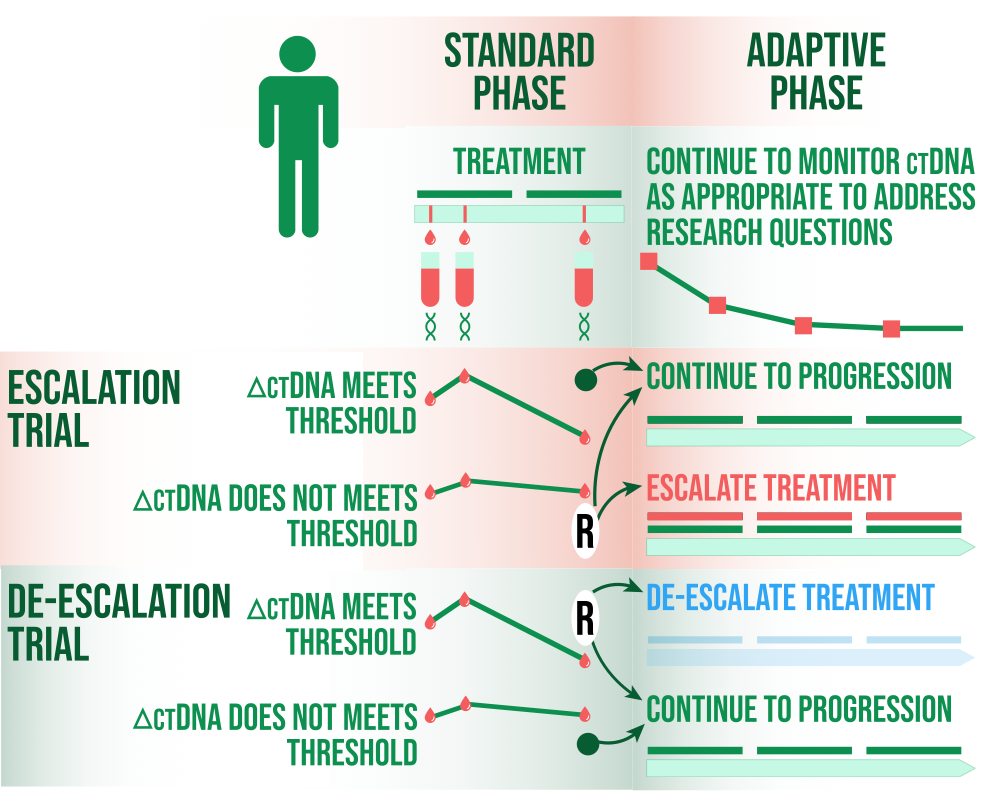
TRIAL Patient Selection
After (neo)adjuvant therapy, ctDNA can be used as a marker of MRD to enhance a trial for patients with higher risk disease and increased events of disease recurrence or death. A biomarker-positive population's study eligibility may be determined by ctDNA testing. A study enrolling both ctDNA negative and positive patients could alternatively use the ctDNA status at baseline as a stratification factor. Both the intent-to-treat population and just the ctDNA positive group could be tested using hierarchical testing procedures. Patients with ctDNA positive status (higher risk) may benefit from an escalation design that adds an experimental therapy to standard care rather than standard care alone, or they may benefit from a deescalation design based on ctDNA negative status.
MRD as a Measure of Response
ctDNA as a Response Measurement. Early-stage clinical trials might make use of ctDNA to help identify drug activity signals and support further drug development strategies. E.g. after neoadjuvant therapy, the FDA encourages sponsors to create evidence demonstrating the value of ctDNA response information in addition to or in support of pathologic complete response data.
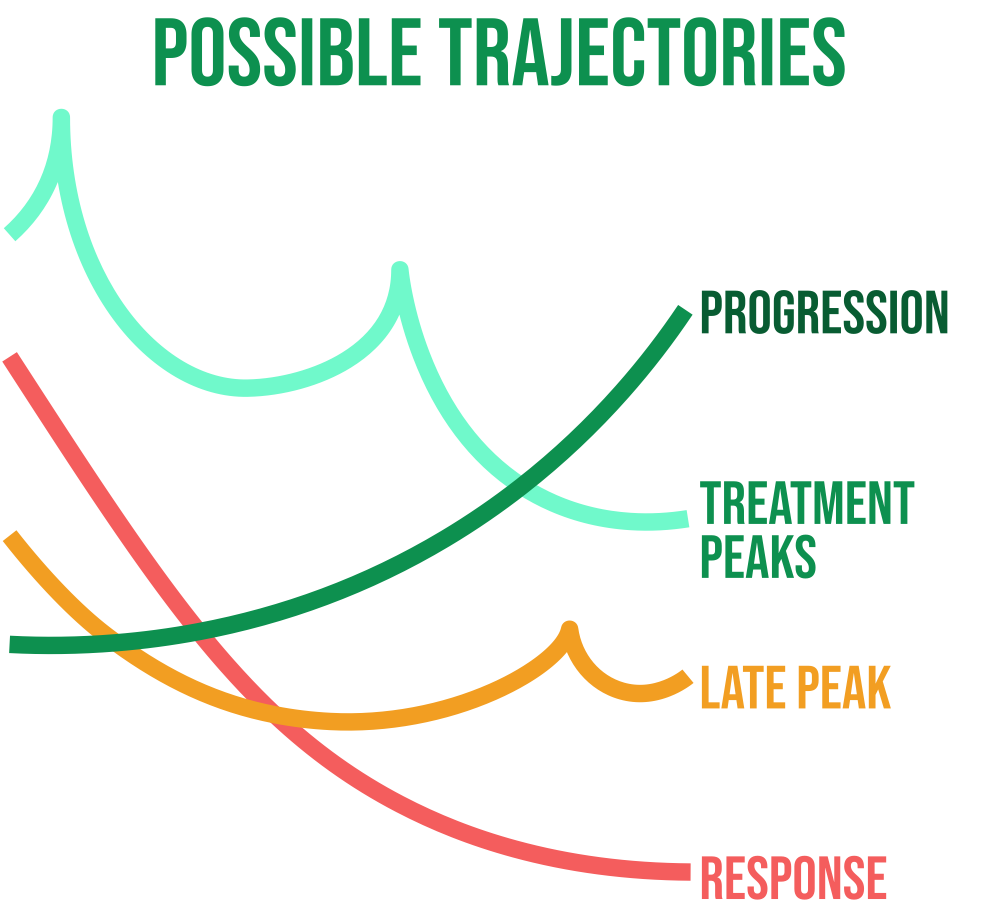
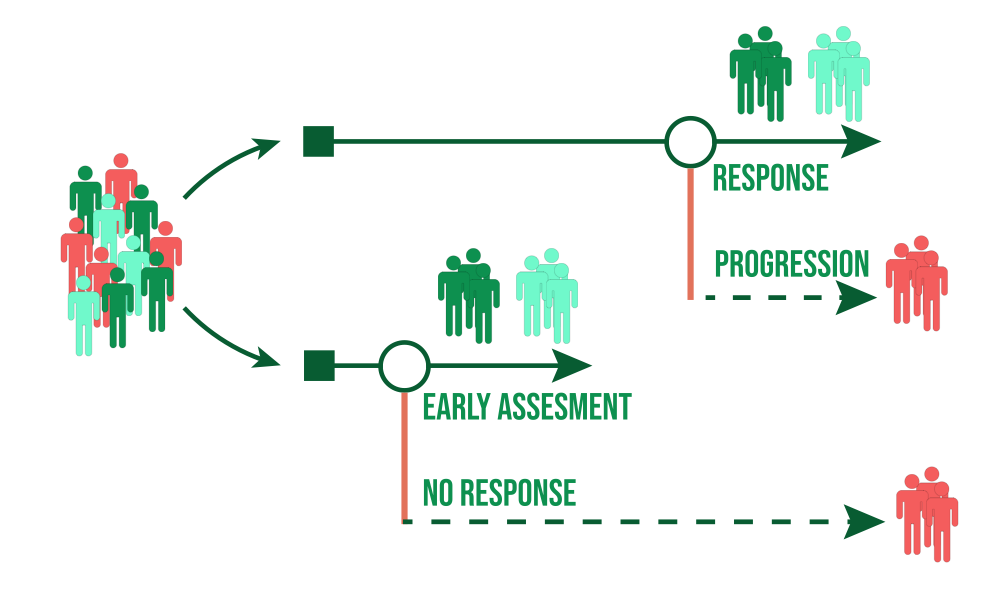
Early Endpoint in Clinical Trials
Changes in ctDNA in response to a drug have the potential to be used as an early endpoint to support drug approval in the context of early-stage cancer, despite not being currently validated for use. ctDNA as an endpoint is likely to predict long-term outcome (DFS/EFS/OS). The relationship between ctDNA clearance and outcome allows trials that collect ctDNA data before and after drug treatment to characterize long-term outcome data. For endpoint validation, a number of statistical criteria have been put forth, and meta-analytical methods were developed.